Regulatory T cell therapy for immune-related diseases
- Field
- Therapeutic Biologics
- Patent
- IP00501
Key Problem and Market Opportunity
- Transplantation-related diseases, autoimmune diseases, and allergy are caused by unwanted responses of the human immune system. Current strategies to treat these diseases rely on the general suppression of the immune system lead to severe side effects such as opportunistic infections and tumor relapse.
- Less than 5% of patients who should undergo bone marrow and organ transplants worldwide can undergo transplantation. It might be no longer necessary to consider human leukocyte antigens (HLA)-match for bone marrow and transplantation if this Treg-based therapy can be successfully translated into the clinic.
Key Advantages of the Technology
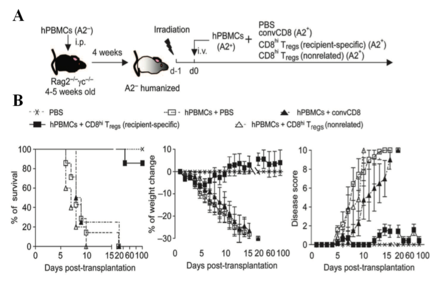
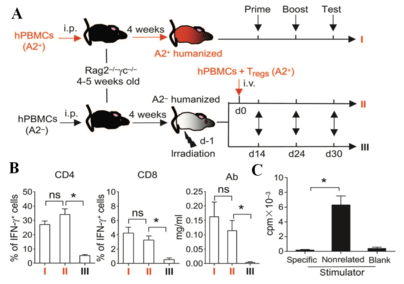
Regulatory T (Treg) cells maintain immune tolerance by suppressing pathologic immune responses in transplant allograft rejection (TAR) and graft-versus-host disease (GVHD). However, conventional CD4+ and CD8+ Treg-based therapies are hampered by their small-scale expansion and unwanted side effects like their global immune suppression. HKU researchers successfully generated a large-scale human allogeneic-specific CD4+ and CD8+ Treg under a simple and cost-effective novel method from naïve precursors ex vivo. These innovations may improve and accelerate the clinical application of Treg-based immunotherapy in bone marrow and organ transplantation, and also has a potential to be used for treating autoimmune diseases and allergy.
Further Details
- DOI: 10.1126/scitranslmed.3004943
- DOI: 10.1182/blood-2008-04-152041
Benefits
- The methods are simple and low-cost
- Personalized therapy
- No need for donor-recipient matching
- Alloantigen-specific suppression:
- induce a long-term tolerance;
- preserve general immunity and graft-versus-tumor (GVT) activity.
Potential Product and Services
- Cell therapy against multiple immune-related diseases such as transplantation-related diseases, autoimmune diseases, and allergy.
Development Status and IP Strength
Patents
- US patent grant No 10,092,597
- US patent grant No 8,658,159
- Chinese patent No ZL200980125228.0
- EP patent No 2300602
IP Status
- Patented